 Harmony
Harmony
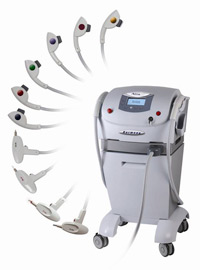 The Harmony® Multi-Application System is the world's most sophisticated, expandable platform for aesthetic laser and light treatments. It uses up to seven distinct technologies built into one compact unit.
The Harmony® Multi-Application System is the world's most sophisticated, expandable platform for aesthetic laser and light treatments. It uses up to seven distinct technologies built into one compact unit.
Features:
- One platform
- Multi-application
- Seven distinct technologies
- Over 60 FDA cleared indications
- Guaranteed upgradability
- Customizable to fit your practice
- Easily transportable
- Depot repair service available in the US
- Affordable
With the advent of Harmony®, physicians can now perform the widest range of applications ever available on a single system.
Harmony's technologies have been carefully selected and refined to not only provide excellent safety and efficacy, but to ensure the highest levels of predictability, reliability and reproducible results.
Modules / Technology
- Advanced Fluorescence Technology (AFTT) - Light-based technology for a wide range of skin treatments including skin rejuvenation, vascular/pigmented lesions, hair removal and acne
- Near Infrared Technology - The near-infrared 780-1,000 module is intended for use for deep dermal heating and is indicated for the treatment of lax skin in areas such as face, neck and underarm.
- Long-Pulse 1064nm Nd:YAG Technology - For the treatment of facial wrinkles, as well as deeper and reticular leg veins
- Q-Switched Nd:YAG Technology - For the treatment of tattoos and dermal pigmented lesions
- Erbium YAG Laser Technology - The module is indicated for minimally ablative resurfacing procedures including wrinkles and scar revision.
- Long-Pulse 1320nm Nd:YAG Technology - The module is indicated for the non-ablative treatment of facial wrinkles, improving the appearance of photo-aged skin.
- Pulsed UVB Technology - For restoration of lost pigment and psoriasis
- Pixel 2940nm Er:YAG Technology - The Harmony module for fractional ablative skin resurfacing
FDA Status
Harmony® was cleared to market on April 5th 2004. The FDA has classified the Harmony® system as a class II medical device.
Learn More:
Brochures and Datasheets:
Module Datasheets:


